User's Manual for the Heat Seal Calculator - Jaws
HSC-J
You can find a short demo video on my YouTube channel.Heat sealing within heated jaws is by far the most common type. In general both jaws are heated but in some circumstances one jaw is kept at room temperature.
The aim is to bring the two heat seal polymers to their seal temperature, TSeal in the shortest possible time with the minimum risk of under-heating (producing a seal that is too weak) or over-heating (producing "polyballs", bubbles, distortion or general degredation). The science is well-described in the standard text on heat sealing by Dr Kazuo Hishinuma, published by DesTech Heat Sealing. Technology and Engineering for Packaging, Principles and Applications.
The app allows you to set the temperatures of the jaws, the thicknesses of the two heat seal layers, the polymer type for those layers, plus an additional two layers between the jaws and the heat seal which might be, for example, a PTFE coating on the jaws and/or a temperature-resistant PET layer. If you have a more complex structure with multiple layers you will need to approximate them with just two. Although thermal properties are important (and an approximation won't be exact), the overall thickness is the most significant property to get right.
As Dr Hishinuma's book stresses, the most important thing you need to know is the temperature at the heat seal interface. His MTMS (Measuring Method for Temperature of Melting Surface) uses a tiny thermocouple to measure this value directly. But if you can't access such a device, the app is the next best thing. The graph on the right shows how that temperature varies with time. Assuming you know TSeal and TBad (when bad things start to happen to your system) your aim is to set the jaw temperatures so that you reach TSeal in the timescale of your process and that small variations in the clamp time take you neither below TSeal nor above TBad. Given that all operators tend to increase the jaw temperature ("just to be safe") it is vital that you use the app to see what happens. A higher temperature will certainly get the system to TSeal more quickly, but the question is whether it will then put you into the region of TBad.
The graph on the left shows the temperature distribution from top to bottom, jaw to jaw (silver), colour-coded in time from violet (short time) to red (long time). It is a great way to see what is happening at the different layers within your system, which are marked out in horizontal lines.
The app does not have a "Pressure" option. This is because pressure makes no difference to heat sealing once you are above a minimum pressure that ensures perfect contact between the two heat seal surfaces. This minimum pressure depends on the roughness of the surfaces and on how well the two surfaces are brought together. If you have to use high pressures to get a good seal then it's likely that the overall system is sub-optimal. The book suggests a pressure of 0.1-0.2MPa. To put things slightly differently, the fact that there is no "Pressure" option means that the model assumes near-perfect thermal contact with the jaws and that the heat flow from the jaws to the polymer is exactly matched by the jaw's heater - i.e. that the jaw temperature remains constant. If you don't have a constant jaw temperature then, once again, your system is sub-optimal. The best systems are able to measure/control the temperature of the jaw at a point very close to the contact point, and the app assumes that this is the case.. What is the meaning of "near-perfect"? Between any two surfaces there is always a Thermal Contact Resistance (TCR). This means that the amount of heat that can flow is less than might be theoretically calculated from temperature differences - especially at the start of the process when the hot jaws touch the cold plastic. The TCR arises because no surface is perfectly smooth so there will always be air gaps (however small) between the surfaces.
Calculating/estimating/measuring TCR is very hard and for this app a pragmatic/pessimistic value is used of 0.003 m²s/W. This is towards the high (bad) end of measured aluminium/plastic interfaces (but there is a very wide scatter in the literature) but may well be realistic in a production environment where the jaws aren't always kept as a highly-polished surface and the polymer films often are micro-rough to enable handling. If there is a user consensus that this value is too low then it can be changed in future versions. For those who want the highest-speed sealing, then reducing contact resistance via a highly-polished jaws and smooth polymer is one way to go. For example, in testing the app, for a case where the seal reached 130°C in 0.2s with the 0.003 TCR it reached that temperature in 0.14s with a TCR of 0.001.
At TBad there are multiple problems. The first is the formation of "polyballs". The heat seal layer becomes too liquid and the pressure of the jaws forces liquid out of the seal, producing a ball of polymer at each end of the seal. This looks unsightly and is also a weak point for a seal. The second problem comes when volatiles are emitted, causing bubbles at the heat seal interface. The third problem is thermal degredation of the polymer. The fourth problem is general distortion/crinkling that looks bad and can also cause tear failures.
So far we've discussed TSeal as if it is a simple idea. But there are two forms of seal. When you want an indestructible seal then you just need to be a bit above TSeal and all will be well. But when you want controlled peelable seal life is much more difficult.
Types of peel
When polymers are allowed to melt together they become "entangled". Although this is a common word to describe tangled bits of string or wool, in polymer physics it has a precise meaning. At the "critical entanglement length" of overlap of chains, melt viscosities soar to a high level, reptation rather than Roussian dynamics takes place and, importantly for us, the strength of an entangled bond is indistinguishable from that of the polymer itself. Entanglement is sudden. If polymer chains intermingle a small amount then you get "de Gennes" type "nail" adhesion, when they become entangled, adhesion rises rapidly. For an in-depth look at these issues visit my Practical Adhesion site.So, as stated above, it's quite easy to get full, tearable adhesion provided the temperature at the interface exceeds TSeal. The problem is that for peelable seal de Gennes (nail) adhesion is proportional to the amount of overlap between the polymer surfaces and at these temperatures it takes very little time for the overlap to increase significantly. So it is very important to reach just below TSeal for a short time before the jaws open. This is very hard to achieve.
The trick for peelable seal is to design a polymer that doesn't much like to get entangled and which is also relatively weak. It then becomes easier to hit a sweet spot of controlled peel.
What is TSeal?
In principle TSeal and Tm the polymer's melting point should be the same. The key issue is that many heat seal polymers such as PE will, under very careful analysis, show multiple values of Tm. Therefore TSeal is an experimental value for a given polymer that is somewhere between the lowest and the highest values of the various Tm.
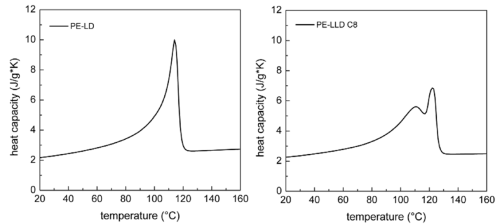 To model the effects of melting we could absorb heat via two methods: heat capacity and enthalpy of fusion. But a more elegant approach from Bach (see below) is to use a variable heat capacity which increases strongly around the melting zone.
For the app the heat capacity curve assumes two forms for each meltable polymer PE and PP. The N(arrow) form (on the left of the figure) has a relatively sharp and large increase in heat capacity (i.e. melting is over a narrow temperature range of ~15°) and the W(ide) form has a less sharp and smaller increase in heat capacity (i.e. melting over a wide temperature range of ~30deg). The area under the curves are the same (so the enthalpy of fusion is the same), but the effects on the temperature/time curves are visibly different. It is a common observation that Wide polymers are easier to seal reliably (given normal process variations) than Narrow ones. Of course such curves when measured in a DSC are scan-speed dependent. The curves here are relevant to the timescale of typical heat sealing.
To model the effects of melting we could absorb heat via two methods: heat capacity and enthalpy of fusion. But a more elegant approach from Bach (see below) is to use a variable heat capacity which increases strongly around the melting zone.
For the app the heat capacity curve assumes two forms for each meltable polymer PE and PP. The N(arrow) form (on the left of the figure) has a relatively sharp and large increase in heat capacity (i.e. melting is over a narrow temperature range of ~15°) and the W(ide) form has a less sharp and smaller increase in heat capacity (i.e. melting over a wide temperature range of ~30deg). The area under the curves are the same (so the enthalpy of fusion is the same), but the effects on the temperature/time curves are visibly different. It is a common observation that Wide polymers are easier to seal reliably (given normal process variations) than Narrow ones. Of course such curves when measured in a DSC are scan-speed dependent. The curves here are relevant to the timescale of typical heat sealing.
The summary of all this is that a polymer shown as PE:105N is a low density, controlled branching PE with an effective TSeal of 105°C and a Narrow melting window, PE:105W has a different form of branching giving a Wide window around the same nominal melting point - and so forth for the other PE and PP entries. Future versions of the app might allow these temperatures and width to be input variables.
Optimising
For speed a high temperature is best, but raises the problem of oversealing (which is the most common fault in the industry).For security, a lower temperature, a few degrees above TSeal will give reliability with no risk of oversealing, but at the cost of slow speed.
The real optimum is the combination of a temperature lower than Tbad and layers as thin as possible. For sealing, anything more than, say, 10nm is a waste of polymer, energy and time. You can't get a more perfect seal than 10nm of entanglement, and for peelable seal you just need a few nm. Some experiments with the MTMS system will quickly tell you what TSeal is, and with the thinnest possible heat seal layers you will get all the seal you need in the shortest possible time with no danger of oversealing. Psychologically this is hard to sell - a thick heat seal layer seems somehow to be more reliable. But in terms of the seal itself (there may be other reasons for choosing a thick layer) a few μm is more than sufficient.
A classic optimization example in the book discusses the use of PTFE on the jaws. It is generally assumed that this is a "good thing" as it should stop damage to the film. But by playing around you will easily find that it can make matters worse because in order to regain the speed lost because of the extra thickness of PTFE, the jaw temperature has to be higher which risks taking the seal above TBad. With the Fixed PTFE option selected, the PTFE on the jaws is assumed to be at jaws temperature at the start of the process - and is not there to slow down cooling on separation.Even with this assumption, the PTFE significantly slows things down because of its low thermal conductivity compared to the jaws.
Details of the calculation
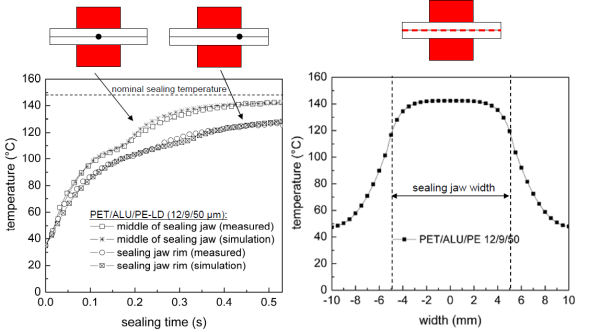
The app uses a classic "finite difference" calculation using 4μm thick "slices" through the polymer sandwich and short timesteps during which the heat flows and temperature changes are calculated. [Using 1μm slices is acceptable on a fast computer but too slow for iPad users] Heat flows into each slice according to the thermal conductivity of the slice and the temperature difference from the previous slice. Heat flows out of the slice via a similar process. The net heat flow is then used to raise the temperature according to the heat capacity of that slice. As discussed above, the heat capacity is temperature dependent with Narrow or Wide increases in heat capacity around the nominal melting point. The representative curves used are modelled on real data kindly provided by Sascha Bach at Technische Universität Dresden. Bach's work (see for example his paper Coupled Finite-Element Modelling of Heat Sealing and Ultrasonic Sealing Processes for Multilayer Packaging Films, 13th TAPPI European PLACE Conference, Bregenz, 2011, images used with permission) also elegantly shows that edge effects are not too important for normal seals, but if they contain layers of Al then lateral conductivity is sufficiently large to cause real edge problems. These extra effects are not described in the app but an example from Bach is shown below.
The MPts of PTFE, PET and PA are assumed to be infinite for the purposes of these calculations - the aim of the app is to melt just the PE or PP.
The big problem with such calculations is speed. If the timestep is very short there are no numerical artefacts but the calculation is slow. If the timestep is too long then the numerics explode. As is common, a short timestep is used near the start when there are large heat flows, with a steadily increasing timestep in later steps to give a speed boost.
Cooling curves
Again the advice of and data from Sascha Bach at Technische Universität Dresden is gratefully mentioned. Cooling once released from the jaws is governed by convection. Without forced air flow convective cooling in air is relatively slow and depends on the orientation of the object. If it's vertical then the heat transfer coefficient is equal on both sides and is relatively large because the hot air can rise, dragging cool air behind it. If it's horizontal then the top surface cools faster than the lower surface as convection is hampered beneath the object.But the rate of cooling gets slower quite rapidly. First, of course, the rate of cooling depends on the temperature difference between the object and the air. But second, the convective heat transfer coefficient also decreases as there is less convective flow introduced at lower temperatures. So the calculations involve both the temperature dependence and orientation dependence of the object! In reality, some extra airflow is likely, leading to faster cooling. For example, at 140°C for the vertical orientation the HTC is around 10W/m²K and at 60°C it is around 7. With forced air these numbers could easily be doubled.
And there's another effect! The heat absorbed by the melting polymer gets emitted during cooling. But crystallisation during cooling takes place at an offset from the melting temperature. In principle one could apply the Avrami equation and other more sophisticated ideas, but for simplicity a fixed offset of 30° from the MPt is used.
Who cares about the cooling rate? It depends on your hot tack. If it is high then the seal can survive subsequent steps, if it is low then subsequent steps have to be done with much more caution
Given the complexities, please use the cooling calculations with caution. They have been tested on a small number of datasets. If anyone would like to provide some extra data that would be much appreciated.
Reality Check
I had the extreme good fortune to meet an expert on heat sealing in the real world, Kevin Chandler from QualityByChoice. He has taken HSC-J for a test drive and here are his conclusions:- The timescales are about right, so the basic calculation seems reasonable.
- The theory is correct about TCR and beautifully polished Al jaws should work much better than the ones found in reality. Good preventative maintenance is to clean the jaws say once a week. But the practical reality is that there is no obvious slowing down when the jaws get a bit dirty. There is no obvious explanation for this, though it raises a question.
- Is radiant energy from the jaws a significant part of the heating effect? My rough analysis suggests that the 7μm radiation at these sorts of temperatures will not be sufficiently absorbed to make much of a difference. If any reader has information either way, please let me know.
- Although double-sided heating is desirable, it can be difficult to get perfect compliance of the jaws when they are rigid. So a rubbery second side can do a reasonable job. The app doesn't simulate the presumed warming up of the rubbery second side, nor the low thermal conductivity which will not suck as much heat from the seal as is suggested by the app.
- "Horizontal" seals that work between two heated rollers have a very short contact time which shouldn't (according to the app) be long enough. However, pre-heating with IR lamps is often used and the system seems to work OK if designed carefully. I assume that these lamps are at significantly higher temperatures than a typical jaws, so my reservations about sufficient radiant energy mentioned earlier are no longer relevant. If, for example, the radiant element were at 1000°C there is plenty of 2μm radiation that is readily absorbed by PE.