Acid/Base Constants
Quick Start
In addition to the Dispersive and Specific (polar) parts of the surface energy, you can measure the acid/base characteristics.
The specific interaction parameters Isp can further be interpreted if a wide range of probe molecules of different electron acceptor (AN) and donor numbers (DN) are being used. These numbers are based on the semi-empirical scale of Gutmann (1978), corrected by Mukhopadhyay P., Schreiber (1995), and describe the Lewis acid-base properties of the probe molecules.
The ISP of a probe is related to the known acid-base constants DN and AN of the gas probe and to the unknown acid-base constants KA and KB of the solid:
ISP=DN.KA+AN.KB
This relation describes the interaction between two amphoteric partners. Nicely, the values of the unknowns KA and KB can be determined by transforming the previous equation into:
ISP/AN=DN/AN.KA+KB
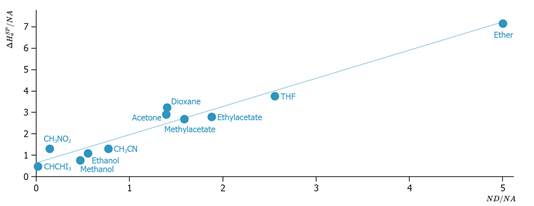 An example of application of this method to the determination of the acid-base character of two celluloses is shown here.
An example of application of this method to the determination of the acid-base character of two celluloses is shown here.
Usually, a satisfactory linear correlation is obtained if many probes with significantly different AN and DN values are used. The slope represents thereby the acid surface constant Ka and the ordinate the basic surface constant Kb.
The Ka and Kb values are a clear indication of the acid-base, or better the electron donor-acceptor properties of different solids. The strength of an amphoteric property is also apparent.
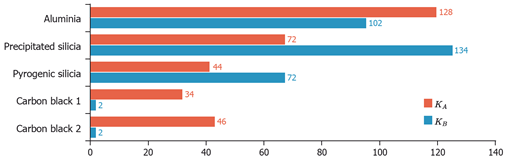 In this example, carbon blacks are mainly acidic and only very weakly basic. The pyrogenic silica presents a major acid (basic ??) character attributed to the presence of surface silanol groups. The precipitated silica, which is more hydroxylated, exhibits higher acid-base constants. Finally, the aluminium oxide that carries important basic and acid sites, has clearly a more pronounced amphoteric character.
In this example, carbon blacks are mainly acidic and only very weakly basic. The pyrogenic silica presents a major acid (basic ??) character attributed to the presence of surface silanol groups. The precipitated silica, which is more hydroxylated, exhibits higher acid-base constants. Finally, the aluminium oxide that carries important basic and acid sites, has clearly a more pronounced amphoteric character.
 The IGC apps are based on the inputs kindly provided by Dr Eric Brendlé of
Adscientis who are specialists in IGC measurements.
The IGC apps are based on the inputs kindly provided by Dr Eric Brendlé of
Adscientis who are specialists in IGC measurements.

