Crystallization Map
Quick Start
The classic Crystal Nucleation Theory has proven to be disappointing in terms of understanding the multiple phenomena in reality. By thinking in terms of maps of different landscapes we start to see the possibilities of tight clusters (classic CNT), loose clusters, clusters leading to oiling out and clusters developing inside clusters - the 2-step mechanism. With such mapping, crystallization starts to make more sense across the wide range of phenomena shown here.
Credits
The app was originally inspired by a paper1 by Prof Peter Poole and colleagues at St Francis Xavier U in Nova Scotia that considers crystallization as a landscape for which a map is very helpful. It then grew considerably.
Crystallization Map
Warning: include(apps/js/crystallization-map.v1.js): Failed to open stream: No such file or directory in /var/www/vhosts/stevenabbott.co.uk/httpdocs/practical-solubility/Crystallization-Map.php on line 33
Warning: include(): Failed opening 'apps/js/crystallization-map.v1.js' for inclusion (include_path='.:/opt/plesk/php/8.3/share/pear') in /var/www/vhosts/stevenabbott.co.uk/httpdocs/practical-solubility/Crystallization-Map.php on line 33
The standard story of crystallization has been acknowledged for decades to be faulty - but there is much confusion about what the alternatives are. My view is that the confusion arises from a lack of understanding of crystallization landscapes. We need to imagine that landscape as being full of mountains, passes, saddlebacks, ridges, and the equivalents of watersheds leading to different final resting places.
Although there are an infinity of possible landscapes, here we look at a few.
- Classic CNT. It is clearly the case that some journeys from supersaturated molecules to macro crystals proceeds via a straight path through a simple pass in the mountains to the crystal pastures beyond. Classic CNT describes this well.
- Loose CNT. It is equally clear that the molecules can assemble not only into sub-crystalline nuclei but into loose, mobile clusters, common in other solubility situations such as near-immiscible liquids. Such large clusters can be found by scattering experiments. At some point, they are sufficiently assembled that the molecules inside can start to crystallize (without the large penalty of crystallizing in a pure solvent environment) and the whole system tips over to a crystal form. So this journey is a long, slowly rising side valley that finds itself near the top of a ridge and can cross over to the crystal pastures.
- Heteronucleation. There's nothing special about clusters forming around junk. Once they form, they can carry on crystallizing via any of the variants in the app.
- Oiling Out. This is the loose cluster valley that didn't rise enough to reach the ridge over to the crystal form and, instead, kept going till it found a sudden drop down (spinodal) to a stable end point with an oil containing plenty of solvent.
- 2-Step. We get the loose clusters of the Loose CNT route, but a twist in the landscape allows a different crystal habit to start forming inside the clusters, tipping over a ridge down into a 3rd stable zone.
What do we mean by "large clusters"? In protein crystallization it is common to see "oil" drops in the microscope, with crystallization taking place inside. If you call this 2-step then it is easy to say that 2-step doesn't happen for small molecules because we rarely see such drops. Others believe that clusters are "colloids" and can start to have colloidal interactions with themselves (argued to be not so important) or with seed crystals (argued to be significant). These ideas are not mutually exclusive - they are focussing on different variants of the mountainous landscape.
Crystal growth and seeds
Once you accept clusters, at least three aspects of crystallization make more sense.
- Classic growth. At relatively low supersaturations, maybe we can justify the standard picture of molecules reaching the surface through a depletion zone and just making the crystal grow.
- Classic growth via clusters. Although it's certain that some crystals grow one molecule at a time, many grow via the clusters that assemble on the surface.
- Crystalline clusters. What happens if a crystlline cluster arrives on the surface? There are at least 3 possibilities of the seed just being swept off, of growing and then being swept off, or becoming incorporated via loose clusters.
- Loop macrostep purification. A "loop macrostep" is what most of us would call a blob or cluster sitting on the surface. Such blobs can overwhelm an impurity that's blocking the growth, kicking it out into the bulk.
- Dendritic growth. We're over-saturated and large clusters at the surface just grow too quickly, too disordered, giving dentritic growth.
- Seed clusters. This is the interesting bit. For whatever reason, large clusters assemble on the surface (perhaps via vdW attraction, the core idea from Botsaris back in the 1980s/90s) and, contrary to popular ideas of crystal growth, just sit there not doing much. If there's a burst of fluid flow or anything that can sweep across the surface, these large clusters head off into the solution and start growing their own crystals. I find the arguments for this mechanism compelling, but the literature is confused and there isn't a rational explanation of why they sit as clusters on the crystal surface, yet crystallize once freed from the surface. Watch this space.
- Mesocrystals. One logical possibility is that local mesocrystals form from small clusters, and these attract more mesocrystals, assembling a whole macrocrystal which, by x-ray looks normal but when looked at closely shows the mosaic of not-quite-perfectly aligned mesocrystals. This has been observed with crystals growing in a lab and also observed within samples carefully taken from the giant gypsum crystals in the Naica Mine.
Landscape Simulations
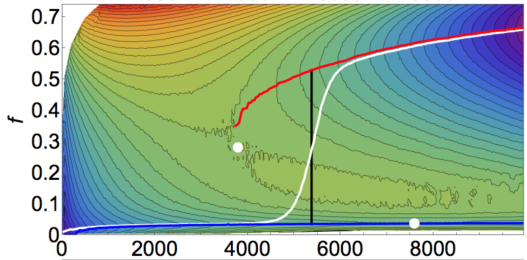 My own instincts, coming with a Kirkwood-Buff approach to solubility issues, are at ease with the landscape idea. Another phrase for KB is "fluctuation theory" (see the Fluctuations app) and I was delighted to find a paper1 by Prof Peter Poole and colleagues at St Francis Xavier U in Nova Scotia that uses fluctuation theory to create the sort of landscapes I'd imagined. Their Fig 3 and its landscape equivalent in Fig 8 b (reproduced here with kind permission of the author) exactly capture the 2-Step path shown, far more crudely, here. The x-axis is the cluster size, the y-axis is the ratio of the second type (gold in my image) to the first type (blue). The white line is the path through the landscape - large blue clusters build up till there's room for the gold clusters to be created and the mountain ridge is overcome.
My own instincts, coming with a Kirkwood-Buff approach to solubility issues, are at ease with the landscape idea. Another phrase for KB is "fluctuation theory" (see the Fluctuations app) and I was delighted to find a paper1 by Prof Peter Poole and colleagues at St Francis Xavier U in Nova Scotia that uses fluctuation theory to create the sort of landscapes I'd imagined. Their Fig 3 and its landscape equivalent in Fig 8 b (reproduced here with kind permission of the author) exactly capture the 2-Step path shown, far more crudely, here. The x-axis is the cluster size, the y-axis is the ratio of the second type (gold in my image) to the first type (blue). The white line is the path through the landscape - large blue clusters build up till there's room for the gold clusters to be created and the mountain ridge is overcome.
A core insight in the paper (and from the references therein) is that unlike standard CNT where there is just one (negative) free energy for cluster formation and one (positive) barrier from surface energy, there are multiple possibilities. For example, in the 2-Step shown, the surface energy of the golden form within the solution might be impossibly high, while that of the blue form isn't too bad, especially in the loose mode. However, the surface energy of the golden form within the blue cluster is not a problem, so it's relatively easy to crystallize from the blue clusters into the thermodynamically more stable golden form.
My view is that a combination of that sort of theoretical mapping with experiments open to the wider notions of clusters within landscapes will bring fresh vitality to the world of crystallization. Surely the curious behaviour of large proto-seeds sitting on a crystal surface, doing nothing till swept away, will be amenable to this sort of mapping. If the crystal is accumulating the proto-seeds via general vdW attractions then there's no logical difference to proto-seeds gathering on junk in the system, i.e. heteronucleation. So much becomes clearer with this sort of approach.
You might well disagree
It is obvious, I hope, that I'm not an expert on crystallization. I very much welcome disagreement and correction. I can either remove this app if it's hopelessly wrong or create better guides to a wider or different range of landscapes.
1Daniella James, Seamus Beairsto, Carmen Hartt, Oleksandr Zavalov, Ivan Saika-Voivod, Richard K. Bowles, and Peter H. Poole, Phase transitions in fluctuations and their role in two-step nucleation, J. Chem. Phys. 150, 074501 (2019);


