Optimal Dispersant Concentration
Quick Start
A really good dispersant will give a monolayer cover of your particles for maximum dispersion efficiency. Calculating that from basic information is tricky, but better than relying on rules of thumb from the industry.
Optimal Dispersant Concentration
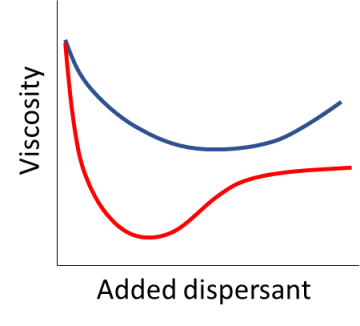 You can measure an Optimum Dispersant Concentration by measuring the viscosity of a dispersion as you add more and more dispersant. At the ODC the dispersant is working to maximum effect and you choose the one with the lowest viscosity at the lowest added concentration. But this is a lot of work, especially if you have to pre-add the dispersant when there is competition from other ingredients in the formulation.
You can measure an Optimum Dispersant Concentration by measuring the viscosity of a dispersion as you add more and more dispersant. At the ODC the dispersant is working to maximum effect and you choose the one with the lowest viscosity at the lowest added concentration. But this is a lot of work, especially if you have to pre-add the dispersant when there is competition from other ingredients in the formulation.
So why not work out the ODC from first principles? The calculations are easy. Knowing the weight, density and radius of your particles you can readily calculate their surface area in m². This is a theoretical area assuming the particles are perfect spheres. If you use a BET surface area measurement, the values are in m²/g of the real material - though how real these values are depends on what the nitrogen is encountering; the surface area inside small pores, for example, is not relevant if the dispersant is too big to get into them. One point of the app is that you can compare the calculated surface area/g to the measured one and draw your own conclusions. Note that the radius used is the Area-Average radius - see the Size Distribution app for an explanation. The alternative, crude measure of surface area using oil (linseed for dibutyl phthalate) gives "g/g" values which aren't easily compared to the real surface area; as everyone says, they are crude guides rather than insightful science.
Similarly, knowing the weight, MWt and effective contact area/molecule of the dispersant you can calculate the surface area available to lock on to the particle. Again, the surface area/g is calculated as m²/mg because some suppliers quote this value or its inverse, mg/m². How do you know the values for any general dispersant? However complex the dispersant, at heart it's going to act as a bunch of heads and a tails joined together. If we assume that the joins are irrelevant we just need the MWt of the core units, and these are going to be like typical surfactants in the 300-600 range, with heads in the 40-60Ų range. If you know that the real MWt is 6000 then you might guess that the head area is going to be 600Ų. As the factor of 10 compared to a 600 MWt monomer cancels out, you might as well assume that you are in the 600 and 60Ų range. If the supplier has quoted a m²/g value, you just choose a MWt and change the head area till you get that value appearing.
The point of all this is to get the % coverage from your dispersant. If it's way below 100% you have unprotected particles. If it's way above 100% you have the risk of depletion flocculation or some other interference with the dispersant effect. If your ODC is way above the minimum required to coat the particle then the dispersant is probably flawed so you should choose a better one via some rational means.


