Crystallization Zones
Quick Start
When you cool a solution in order to get a solute to crystallize many different things can happen, depending on how far and how fast you cool. This diagram describes the multiple possible zones - not all solutes will show all of them!.
Credits
This is part of my series of apps trying to wrestle with the complexities of crystallization.
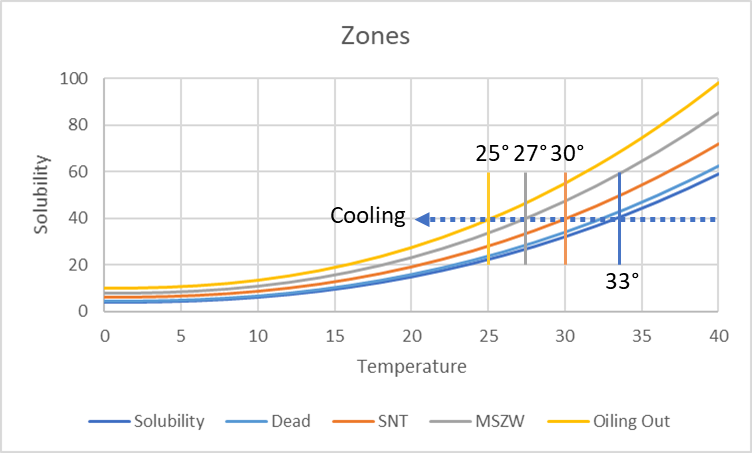 Let's start our solution at concentration and temperature 40 (units undefined as we are showing the principles). At 33° you reach the Solubility curve - this is the formal limit of the solubility of this solute. At, maybe, 32° you cross the Dead zone. In between 33 and 32° even if you have a seed crystal present, nothing happens. You go down to 30° and nothing happens. Even if you put in a seed you don't see anything other than the seed itself growing slowly. Below 30°, the seed might produce Secondary Nucleation, i.e. fresh seeds to that crystals grow throughout the solution, so 30° is the SNT, Secondary Nucleation Threshold.
Let's start our solution at concentration and temperature 40 (units undefined as we are showing the principles). At 33° you reach the Solubility curve - this is the formal limit of the solubility of this solute. At, maybe, 32° you cross the Dead zone. In between 33 and 32° even if you have a seed crystal present, nothing happens. You go down to 30° and nothing happens. Even if you put in a seed you don't see anything other than the seed itself growing slowly. Below 30°, the seed might produce Secondary Nucleation, i.e. fresh seeds to that crystals grow throughout the solution, so 30° is the SNT, Secondary Nucleation Threshold.
If we had no seed and were not especially patient, then we would have to cool to 27° before we started to see crystals appear spontaneously. Up to then, the system was Metastable, so the width of the zone from the solubility curve to this point is the MSZW, Metastable Zone Width.
Even beyond the metastable zone, crystallization isn't guaranteed. If you go even colder, below 25° the solute might just do a liquid-liquid separation (at a spinodal point: see the Metastable Zone app) so you have Oiling Out. The oil, which might be, say, a 60:40 solute:solvent mix might be stable forever or might slowly start to crystallize.
It is worth repeating that for your specific system the zones may or may not appear and may be different relative widths. What you see depends often on your patience and cleanliness. Slow and fast cooling will show different zones. Solutions that have been carefully filtered, in apparatus that has not seen these crystals before will also show different zone behaviours. That's one of the many reasons why crystallization is so frustrating.


