Effective Alkane Carbon Number
Quick Start
To use HLD we need the oiliness of our oil, its EACN. We might be able to find it in the list below. We might be able to measure it. But it it's a mixture, or if we want to tune the oiliness rationally, then we need to calculate the value of the mixture. Easy! It's the volume-based1 average of the individual oils.
EACN
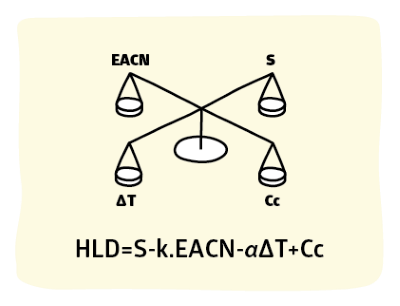 We need to be able to characterise each oil with a number in order to do the HLD calculations. For hexane, heptane, octane etc. it is easy to assign a number: just use the number of carbon atoms in the oil, so 6, 7, 8, ...
We need to be able to characterise each oil with a number in order to do the HLD calculations. For hexane, heptane, octane etc. it is easy to assign a number: just use the number of carbon atoms in the oil, so 6, 7, 8, ...
But if you take cyclohexane which has six carbons it doesn't behave at all like hexane. In fact it behaves as if it has just 3 carbons. Of course it doesn't behave like propane gas (which has 3 carbons), but in all calculations, if you say that cyclohexane has an Effective Alkane Carbon Number = 3 everything works out fine. If you take benzene which still has 6 carbon atoms, it behaves like an oil with 0 carbons, its EACN = 0. And some chlorinated solvents behave as if they have negative numbers of carbons. Dichlorobenzene with 6 carbons and 2 chlorines has an EACN = -5.
One attempt to estimate EACN from a structure is provided at the excellent Pirika EACN estimator site, using Hansen Solubility Parameters (an estimator is also included in Hansen Solubility Parameters in Practice) at the heart of the estimation. But the scheme is based on correlations of data of variable quality and is only a general guide.
We often don't have a single oil. Fortunately, it is easy to blend oils to form a new EACN value, using the (% volume1) weighted average of the two values, as calculated above.
Here is a list of EACN values, updated October 2022. Most of them are reproduced, by permission, from the wonderful 2022 paper from U Lille: Lucie Delforce, François Duprat, Jean-Luc Ploix, Jesus Fermín Ontiveros, Valentin Goussard, Véronique Nardello-Rataj, and Jean-Marie Aubry, Fast Prediction of the Equivalent Alkane Carbon Number Using Graph Machines and Neural Networks, https://doi.org/10.1021/acsomega.2c04592:
| Ambrettolid | 1 | Hexadecane | 16 |
| Asphaltenes | 1 | Hexadecyl acetate | 5 |
| Benzene | 0 | Hexamethyldisiloxane | 12 |
| Bis (2-ethylhexyl) Adipate | 9.7 | Hexane | 6 |
| 1-Bromo-2-methylpropane | -3.3 | Hexyl dodecanoate | 9.3 |
| Butyl dodecanoate | 7.2 | Hexyl methacrylate | 0.1 |
| Butylbenzene | 0.4 | Hexyl octanoate | 6.2 |
| Butylcyclohexane | 7.3 | β-Ionone | -1.9 |
| Canola, Soy, etc. | 18 | Isoamyl laurate | 8.8 |
| Carbon Tetrachloride CCl4 | 0 | Isododecane | 11.7 |
| Δ-3-Carene | 2.5 | Isohexadecane | 13.9 |
| D-Carvone | -3.1 | Isopropyl myristate IPM | 7.3 |
| Caryophyllene | 6 | Isopropylcyclohexane | 5.3 |
| Cetiol-S | 17 | Limonene | 1.8 |
| 1-Chlorodecane | 3.5 | Limonene (alternate) | 7 |
| 1-Chlorododecane | 5.6 | Linalyl acetate | -0.9 |
| Chloroform | -14 | Longifolene | 7 |
| 1-Chlorohexadecane | 9.8 | Maltenes | 6 |
| 1-Chlorotetradecane | 8 | p-Menth-2-ene | 3.4 |
| Corn Oil | 16 | p-Menthane | 5.8 |
| p-Cymene | -0.8 | Menthone | -1.5 |
| cis-Decalin | 5.3 | Menthyl acetate | -0.1 |
| Citronellyl acetate | -0.2 | Methyl cedrylether | 3.5 |
| Cyclodecane | 5.6 | Methyl dihydrojasmonate | -1.7 |
| 1,3-Cyclohexadiene | -3.1 | Methylcyclohexane | 3.2 |
| 1,4-Cyclohexadiene | -4 | 1-Methyl-1-cyclohexene | 0 |
| Cyclohexane | 2.1 | 3-Methyl-1-cyclohexene | -0.5 |
| Cyclohexene | -1.2 | 4-Methyl-1-cyclohexene | 0.1 |
| Cyclooctane | 4.1 | Methylene chloride | -14 |
| cis-Cyclooctene | 1.5 | 2-Methylpentane | 6.4 |
| α-Damascone | -1.3 | 3-Methylpentane | 5.2 |
| cis-Decalin | 5.3 | Myristyl propionate | 6.8 |
| Decane | 10 | Naphthalene | 1 |
| 2-Decanone | -2.1 | Nonadecane | 19 |
| 1-Decene | 5.5 | Nonane | 9 |
| Decanenitrile | -0.5 | 2,5-Norbornadiene | -3.2 |
| Decyl butyrate | 5 | Octadecane | 18 |
| Decylbenzene | 6 | 1-Octadecene | 14.2 |
| Decylcyclohexane | 14.4 | Octane | 8 |
| 1-Decyne | 0.1 | Octanenitrile | -1.7 |
| 1,2-Dibutoxyethane | 1.7 | 2-Octanone | -3.4 |
| Dibutylether | 3 | 1-Octene | 3.9 |
| o-Dichlorobenzene | -5 | Octylbenzene | 4 |
| Diesel | 13 | Octyloctanoate | 8.1 |
| Diheptylether | 8 | 1-Octyne | -1.8 |
| Dihexylether | 6.2 | Paraffin | 18 |
| Diisopropylether | 0.6 | Pentadecane | 15 |
| 2,3-Dimethylbutane | 4.8 | Phenyl-1-butyne | -3.3 |
| 1,2-Dimethylcyclohexane | 3.3 | Pinane | 4.1 |
| 1,4-Dimethylcyclohexane | 4.6 | α-Pinene | 3.5 |
| Dioctylether | 10.3 | β-Pinene | 2.2 |
| Dipentylether | 4.2 | Pristane | 17.6 |
| 1,2-Dipropoxyethane | 0.4 | Propylcyclohexane | 5.9 |
| 1,4-Dipropoxybutane | 1.9 | Rose oxide | -1.7 |
| Dipropylether | 0.4 | Squalane | 24 |
| Dodecane | 12 | Squalene | 16 |
| Dodecanenitrile | 0.4 | Styrene | 3 |
| 1-Dodecene | 8.1 | α-Terpinene | 1.2 |
| 2-Dodecanone | -0.6 | γ-Terpinene | 1.7 |
| Dodecylbenzene | 7.8 | Terpinolene | 0.7 |
| Dodecylcyclohexane | 17.5 | Tetradecane | 14 |
| 1-Dodecyne | 2 | 1-Tetradecyne | 3.9 |
| Eicosane | 20 | Toluene | 1 |
| Ethyl decanoate | 2.1 | Tricaprin | 13 |
| Ethyl dodecanoate | 3.8 | Trichloroethylene | -3.8 |
| Ethyl myristate | 5.3 | Tridecane | 13 |
| Ethyl oleate | 7.2 | Trilaurin | 16 |
| Ethyl palmitate | 6.8 | 2,2,4-Trimethylpentane | 8.3 |
| Ethylcyclohexane | 4.2 | 2,6,10-Trimethylundeca- 2,6-diene | 10.3 |
| Ethylene brassylate | -1.1 | Trimyristin | 19 |
| Eucalyptol | -1.6 | Triolein | 22 |
| Geranyl acetate | -0.6 | Tripalmitin | 22 |
| Glycerol tridecanoate | 14 | Tristerin | 25 |
| Glycerol trioctanoate | 12.3 | Undecane | 11 |
| Hemisqualane | 14.8 | 2-Undecanone | -1.3 |
| Heptadecane | 17 | p-Xylene | -2.4 |
| Heptane | 7 |
Now you are clear about EACN values, click on the Cc link to learn about Cc values for individual surfactants.
1For many years this page said "weight based". The original paper from Acosta that expressed an opinion said "volume based". In Jan 2020 I did an analysis of an interesting dataset from the famous Prof Strey and found that although mass-based was not bad, volume-based was definitely better. Molar-based in this case was wildly bad because the EACN mix was with triolein and decane!


