Hydrophilic Lipophilic Difference
Quick Start
HLD is about balancing the whole system of water, surfactant, oil, temperature and salts - the task that formulators really face.
If you know the temperature T, salinity S, the oiliness EACN of the oil and characteristic of your surfactant Cc then you can tell where you are in terms of getting the balance you need for your formulation. When HLD=0 you have balance. This may not be where you want to be, but it's a unique reference point in surfactant space.
Updated Aug 2021 with variable k and S=0 for "other" surfactants
HLD
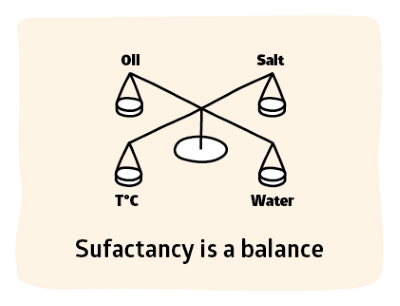 HLD is what HLB should have been: a generally useful, scientifically meaningful approach to surfactant formulation. Given that we want to use our surfactant most efficiently, we want to ensure that the head and tail are nicely balanced within the whole formulation so that the surfactant resides mostly at the interface.
HLD is what HLB should have been: a generally useful, scientifically meaningful approach to surfactant formulation. Given that we want to use our surfactant most efficiently, we want to ensure that the head and tail are nicely balanced within the whole formulation so that the surfactant resides mostly at the interface.
At the point of perfect balance there is no difference between the hydrophilic tendency and the lipophilic tendency so the HLD = 0. The starting point for all surfactant formulations is the identification of the HLD = 0 point. For those who want Type III microemulsions, identifying HLD = 0 is the end of the journey. For those who want conventional emulsions the point where HLD = 0 is absolutely the worst place to be (emulsions will separate with maximum speed!) but by knowing where it is makes it possible to move the formulation to just the right spot to give you a stable Type I o/w or Type II w/o emulsion .
As discussed in Basics, the point of the HLD equation is to juggle the key parameters. Here they are with their usual units:
- EACN - Effective Alkane Carbon Number, i.e. the oiliness of the oil
- T - the Temperature, in °C
- S - the Salinity g/100ml
- Cc - a Characteristic value for the hydrophobic/philic nature of the surfactant
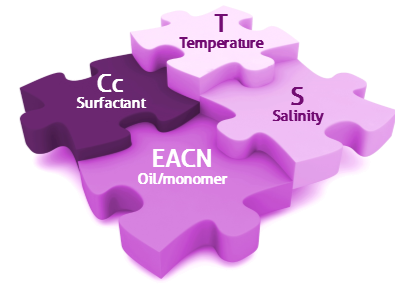 Another way to look at this is to think of the four components as part of a jigsaw puzzle - they need to be locked together for success. I love this image, used with permission from VLCI in Amsterdam who are experts at measuring EACN and Cc values and using those numbers to help clients apply HLD-NAC theory for improved formulations.
Another way to look at this is to think of the four components as part of a jigsaw puzzle - they need to be locked together for success. I love this image, used with permission from VLCI in Amsterdam who are experts at measuring EACN and Cc values and using those numbers to help clients apply HLD-NAC theory for improved formulations.
Those who formulate with alcohols (calling them "co-surfactants") will be disappointed to see them excluded from this list. There are 2 reasons. First, formulation with alcohol is often a desperate measure in the face of the wrong choice of surfactant. Second, there are not enough data out there to include in any workable model as the effect of each alcohol is different.
The HLD Equation
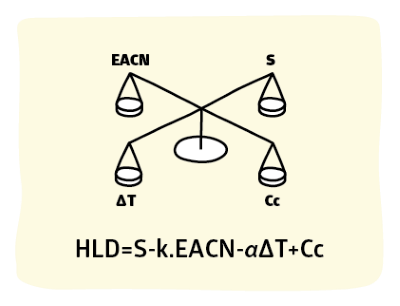
HLD = F(S) - k.EACN - α(T-25) + Cc
This tells us that:
- as S increases, HLD increases; the F(S) means "a function of Salinity" - for ethoxylates it is 0.13*S, for "other" (typically Spans and APGs) there's basically no S-dependence, and for ionics it is ln(S+SurfSal). Because an ionic surfactant adds to the salinity (the rule of thumb is that it is equivalent to 30% of its molar equivalent to NaCl) the % Surfactant and its MWt combine to create SurfSal. The new total salinity is shown in the Total S output box. Use this value in other apps.
- as EACN increases (more oily) HLD decreases (the factor k typically varies from 0.15-0.17 depending on the surfactant, but for extended surfactants it's ~0.06). Use 0.16 as a default
- For ionics, α=0.01, for ethoxylates α=-0.06 and for sugar surfactants such as APGs, α=0. So as T increases above the reference value of 25°C HLD decreases by 0.01 for each degree for ionics, increases by 0.06 for each degree for ethoxylates and is unchanged for APGs
- A high Cc means a large HLD
Yes, but what does it mean?
Let's look at the same equation in a different way. Suppose HLD = 0. Then:
k.EACN = F(S) - α(T-25) + Cc
So for a very oily oil (high EACN) you need either a high salinity (salt drives the surfactant towards the oil), a high Cc (a hydrophobic surfactant) and for an ionic you need a low temperature (because high temperatures increase water solubility) and for ethoxylates you need a high temperature (water solubility decreases with increasing temperature). But anyone who works with surfactants knows that! Of course. But the point is (a) that now you see that the equation fits in with your intuitions and knowledge and (b) you have an actual equation to do the calculation.
Let's see it in action. Choose a type of surfactant (ionic, ethoxylate, other) to automatically give you the correct F(S) and the correct value for α. Now change the other values and see what happens to the HLD.
Although the equation is simple, if you are like most of us it still takes some effort to get used to what happens as you change each parameter. You can get quite easily lost in surfactant space. That makes a very powerful point. Getting lost in surfactant space when you have a formula is not too bad. What is really bad, and all too common, is when you get lost in surfactant space on the formulation bench. The point of HLD is for you to explore surfactant space before you go to the bench, so your bench work will be much more effective.
Before exploring more about HLD we need to be clearer about assigning numbers to oil. Click on the EACN link to discover more.


