Uses
Quick Start
HLD isn't just a good theory; it's useful (there are lots of things you can do with it) and usable (it's not hard to use it to do those useful things).
This page summarises what it can do. Subsequent pages look in more detail.
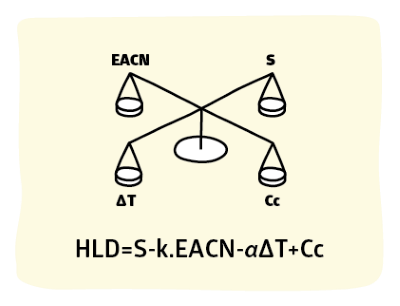 This section focuses on the use of HLD-NAC theory.
This section focuses on the use of HLD-NAC theory.
Microemulsions
This section covers three classic examples of microemulsions:
- EOR, Extended Oil Recovery, which funded a lot of the work on HLD because oil companies depend on this science to extract oil from older wells. $Millions depend on the theory being correct.
- Cosmetic microemulsions. Although these are a small part of the cosmetics emulsions industry they are significant and of increasing interest. As some classes of surfactant used for microemulsions go out of fashion (e.g. ethoyxlates) formulators need a rational approach to enable them to find alternatives. HLD-NAC works very well for this
- Making nanoparticles. Take a w/o microemulsion of, say, barium chloride and mix it with a w/o microemulsion of, say, sodium sulfate, the two react to create nanoparticles of insoluble BaSO4. This is an excellent way to create highly stabilised (lots of surfactant shell!) very small (a few nm) nanoparticles with surprisingly little effort - provided you can used HLD-NAC to get into the right Type I or Type II space.
Cleaning
It is often thought that surfactants clean by lowering the surface tension. If this were true then one, cheap, surfactant would manage all cleaning jobs. In fact this general surface tension lowering is of relatively modest importance (and comes "free" with just about any surfactant). The key to removing an oil is ensuring that the oil-water interfacial tension is low - in other words that the HLD of that given oil in that particular environment (salinity, temperature...) is very close to zero.
Separation
Whether it is crude oil mixed with water or palm oil from pressings, separating oil from water can be very hard. One excellent way to achieve this is by lowering the interfacial energy to zero - i.e. adding a surfactant to the system which brings the HLD=0. It seems counterintuitive to add a surfactant to separate oil from water, but that's how it's done.
Macroemulsions
As explained earlier, the term Macroemulsion is used to describe "ordinary" emulsions and the "macro" bit is to distinguish them from microemulsions. Because "Nanoemulsions" are simply very small particle conventional emulsions, they are included within the definition of macroemulsions. The confusion is compounded by the fact that Microemulsions aren't micro at all but are truly nano.
The topics discussed are:
- 50:50 oil:water emulsions - unstable-stable-unstable-stable-unstable.
- Efficient o/w emulsions
- Efficient w/o emulsions
PIF - Phase Inversion Formulations
Many emulsion experts love the PIT (Phase Inversion Temperature) method for making emulsions. This happens to be a special case of HLD, but most PIT specialists don't use HLD to analyse the effect. PIT (for o/w emulsions) is limited to ethoxylates which undergo a phase inversion at higher temperatures. But why be limited by temperature and ethoxylates? PIF (Phase Inversion Formulation) uses the whole of HLD space to get a system into the crucial "inversion" zone (in other words the zone where HLD=0) for easy creation of an emulsion (because interfacial energy is low) and then quickly away from that zone (e.g. by adding another surfactant or more water or oil) to provide a stable emulsion. PIF gives you double efficiency:
- efficient use of surfactants because they are in the right HLD zone to give maximum effect
- efficient use of energy because energetic mixing and heating/cooling are not required.


