HLD (Expert Mode)
Quick Start
The HLD page laid out the basics. For those who want a little more power and understand HLD, read on.
HLD Expert
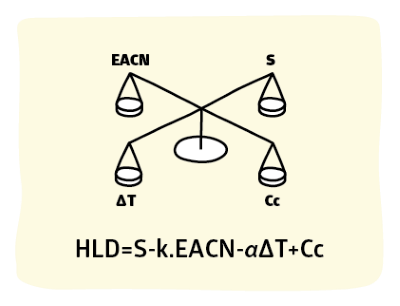 The basic HLD app provides an introduction to calculating HLD. However, expert users require two extra functionalities.
The basic HLD app provides an introduction to calculating HLD. However, expert users require two extra functionalities.
- The ability to choose salts other than NaCl
- The ability to tune HLD via alcohols and "polar oils"
Salts
There are two forms of salt correction. The first takes into account the MWt of the salt. The same weight of LiCl provides more salinity than NaCl and KCl provides less salinity.
The second correction takes into account divalent or trivalent salts. Previous versions of this app used some ad-hoc corrections, but expert advice is to use ionic strength which, of course, corrects for MWt as well as for charges of anions and cations.
Therefore you have to put in the MWt (or atomic weight) of you cation and anion. As a reminder:
- Li=3; Na=23; K=39; Rb=85; Mg=24; Ca=40
- F=19; Cl=35; Br=80; SO4=96; PO4=95; CO3=60
The calculated value (including a nominal 2% correction for ionic surfactants) is shown as Equivalent S.
Alcohols and Polar Oils
At one time it was thought that one could correct for alcohols via some simple factors. However, this has turned out not to be correct. In practice we have to find correction factors for any given alcohol (or polar oil or, even, alcohols within our ethoxylates) via HLD scans. So many people use this sort of formula, but the numbers are specific to each system.
We can rewrite the HLD formula as
HLD = F(S) - k.EACN - α(T-25) + F(A) + Cc
where F(A) is a function of the % Alcohol or other Additive added to the system. It would be wonderful if there were a universal formula for F(A). But currently you have to find your own dependency via HLD scans.
To calculate HLD use the same inputs as in the basic app but choose the MWt of the ions and whether they are mono-, di- or tri-valent. Then enter your % Alcohol/Additive with your estimate of F(A).
If anyone from industry or academia can provide better estimates for the salt or alcohol effects it will be easy to update this app and acknowledge the source of the information! Indeed, this version contains the update to the salt via ionic strength.


